Characterizing a novel pore-forming peptide
My interest membrane proteins and membrane structure lead me the lab of my current PI, Dr. Francisco Barrera. Our lab is interested in understanding biophysical characteristics of membrane proteins that dictate their function in biomedically relevant settings (check us out below).
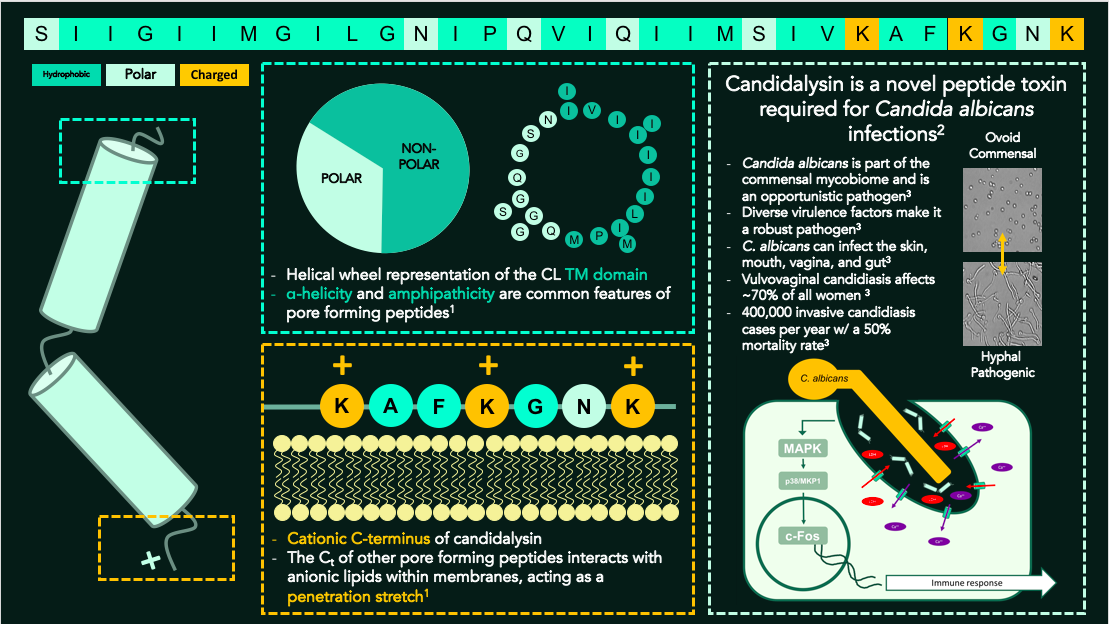
My research is centered around a novel cytolytic peptide produced by the commensal fungus Candida albicans, Candidalysin (CL). It is the first known human pathogenic peptide produced by a fungus and is required for C. albicans infections. CL is an amphipathic a-helix and has a cationic C-terminus. These are common characteristics of pore forming proteins and peptides (PFPs), a family of membrane disrupting proteins. PFPs typically exist as unstructured monomers in solution before adopting a-helical structure and oligomerizing after interacting with the plasma membrane. Membrane disruption can occur via a detergent-like mechanism or through the formation of a membrane-spanning pore. Previous studies suggest CL forms a pore in cell membranes. This is significant because the number of known peptides that form stables pores in membranes is limited. It is also unclear how specific structural features determine pore forming mechanisms.
The goals of my research include identifying specific structural features of CL that allow it to function and understanding how the lipid environment influences its behavior.